The Phase I Clinical Trial Unit of The First Hospital of Jilin University was established in 2011. After 10 years of development, the unit currently has 55 employees and 130 beds, and has developed into a first-class ward (Phase I) in China.
In the past 10 years, the unit has undertaken 353 Phase I clinical trials, including 188 Phase I clinical trials of new drug and 165 trials of bioequivalence and biosimilars. It is the (Phase I) unit that has undertaken and completed the most innovative drug clinical trials in China, which has accelerated the clinical evaluation and achievement transformation of innovative and generic drug in China. Even during the COVID-19 pandemic, the number of projects has not been significantly affected.
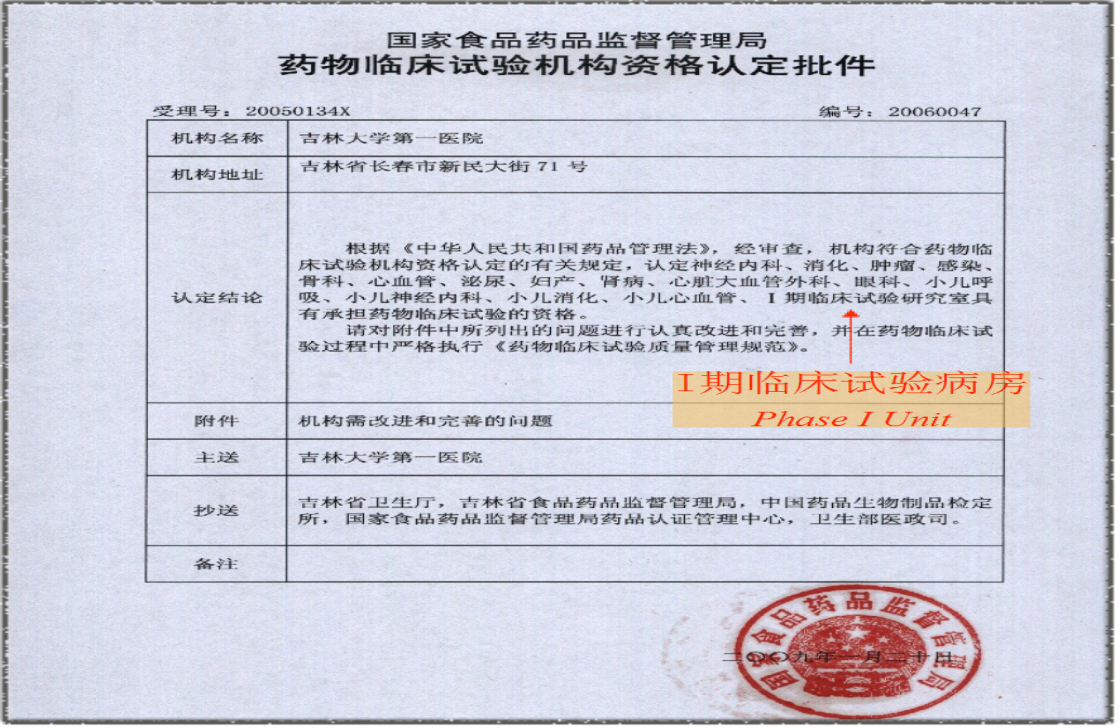
Figure 1. In 2009, the Qualification Approval for Drug Clinical Trial Institutions issued by CFDA


Figure 2. Partner Companies